valency of zinc|Valency : Cebu Zinc is a silvery-white metal with a blue tinge that tarnishes in air. It has a valency of +2 and forms many compounds, such as zinc oxide, zinc sulfide and zinc chloride. Lotto Texas How-to-Play Brochure. STEP 1. Get a Lotto Texas playslip from your favorite Texas Lottery® retailer or create a play using the Texas Lottery® app. . Select six (6) numbers from 1 to 54 in the play area of the playboard or mark the "QP" box and the terminal will select your six (6) numbers.
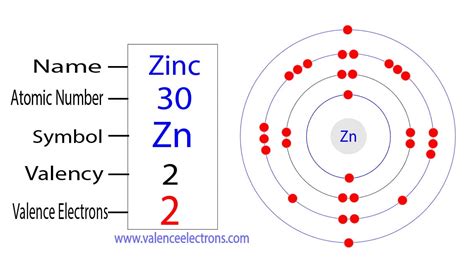
valency of zinc,Dis 24, 2015 — Find the valency of zinc and other elements in the periodic table with this chart. Learn the definition, guidelines and examples of valency and oxidation state of elements.93 rows — Zinc has a valency of +2, meaning it can form compounds with two electrons. .

Mar 24, 2022 — To find the number of valence electrons for Zinc (Zn) we need to look at its electron configuration. This is necessary because Zn is a transition metal (d b.Valency Ene 25, 2021 — Valency of Zinc. The precise and accurate valency of Zinc is +2. Zinc is the metal transition element and hence it belongs to the d block category. The outermost .Zinc is a silvery-white metal with a blue tinge that tarnishes in air. It has a valency of +2 and forms many compounds, such as zinc oxide, zinc sulfide and zinc chloride.Hun 17, 2015 — Learn how to find the valence electrons of zinc by looking at its electron configuration. Zinc has only two valence electrons in the 4s-orbital, which can be lost to form Zn2+ cation.Hun 30, 2023 — Zinc (Zn) is a blue-white metal of moderate strength, hardness and ductility. Zinc is one of the least common elements and is mostly produced through electrolysis of .Zinc is a transition metal with atomic number 30 and valency of 2. It has a silver-gray appearance and is used in brass, galvanizing and alloys. Learn more about its history, .
Valency is the combining power of an element. Elements in the same group of the periodic table have the same valency. The valency of an element is related to how many .The Gas Basicity of Zinc is 586 kJ/mol. The Dipole Polarizability of Zn is 38.67 plus or minus 0.3 a₀. Element 30 has a C6 Dispersion Coefficient (CD) of 284 a₀, and C6 Dispersion Coefficient (GB) of 276 a₀. The Allotropes of Zinc (Zn) is . The Neutron Cross Section of Zinc is 1.1. The Neutron Mass Absorption of Zn is 0.00055.
Hun 17, 2015 — Zinc is located in period 4, group 12 of the periodic table and has an atomic number equal to 30.. This means that neutral zinc atom has a total of 30 electrons surrounding its nucleus. To see how many of .

1 day ago — For example, if the valency of an element is 3, it will share 3 electrons either with a single element also having valency 3 or with three different elements having valency 1. Zinc has a valency of 2. Zinc is a metal and it is electropositive. That means it will readily lose electrons. Since the valency of zinc is 2, it will lose 2 electrons.
Hun 30, 2023 — Zinc is reasonably resistant to corrosion and is used as a covering for baser metals like iron ("galvanizing"). Zinc can be readily cast or molded. Zinc has many unique characteristics. For example, its vapor burns in air with a green flame, forming zinc oxide. Zinc oxide is a common zinc compound that is used in paints, cosmetics, plastics and .
Valency is the combining power of an element. Elements in the same group of the periodic table have the same valency. The valency of an element is related to how many electrons are in the outer shell.
Ago 11, 2023 — Zinc has a less boiling point than iron or copper and hence it was not discovered earlier due to its boiling during the extraction of other metals. Zinc is a hard metal but it can be malleable above 100 °C. Zinc has many isotopes, but out of these isotopes, 64 Zn is the most abundant (around 49%).Zinc is a chemical element with the symbol Zn and atomic number 30. It is a slightly brittle metal at room temperature and has a shiny-greyish appearance when oxidation is removed. It is the first element in group 12 (IIB) of the periodic table.
Zinc's electrical capabilities also extend to the most popular batteries. A traditional dry cell has an outer zinc casing acting as the anode (confusingly the anode, usually thought of as positive, is the negative end of a battery), while a carbon rod provides the cathode, the positive electrode. .Valence Electrons: 4s 2 Electron Dot Model. Chemical Properties of Zinc. Electrochemical Equivalent: 1.22g/amp-hr; . Zinc - Zn (EnvironmentalChemistry.com)- Comprehensive information for the element Zinc - Zn is provided by this page including scores of properties, element names in many languages, most known nuclides and technical terms .Hul 3, 2019 — Zinc has a melting point of 419.58°C, a boiling point of 907°C, a specific gravity of 7.133 (25°C), with a valence of 2. Zinc is a lustrous blue-white metal. It is brittle at low temperatures but becomes malleable at 100-150°C. It is a fair electrical conductor. Zinc burns in air at high red heat, evolving white clouds of zinc oxide.
Ago 28, 2023 — Valency Chart: The number of atoms of one element bound to each atom of another in a molecule is depicted on a valency chart. Valence, or valency, is the. . Valency of Zinc: 2: How to determine the valency of elements? There are three primary methods for determining the valency of any element. The first one is the Octet method, .
Peb 2, 2018 — Zinc is a d-Block element and belongs to Transition Metal. Atomic number of Zinc is 30 and Electronic configuration of Zinc is: \(1s^22s^22p^63s^23p^63d^{10}4s^2\) Valence shell contains 2 electrons that is 4s \(^2\) that means that zinc can lose the two electrons located in the 4s-orbital to be become the Zn \(^{2+}\) cation. So, the valency .
valency of zinc Valency Mar 23, 2023 — For main group elements (i.e s-block and p-block elements), the valence electrons are the electrons present in the outermost orbit. But for most of the transition and inner transition elements, the valence .2 days ago — Explore the valency chart in chemistry, a vital tool for comprehending how elements bond and form molecules. Learn about valence electrons and chemical reactions. . Zinc . 30. 2. Use of Valency. The valency or valency chart is helpful in order to determine how many atoms of an element will combine with another element to form any .May 1, 2022 — Rôle biologique. Le zinc est vital pour tous les organismes vivants. Il forme le site actif de plus de 20 métalloenzymes. Le corps humain moyen contient 2,5 grammes de zinc et consomme environ 15 mg par jour. Le zinc se trouve dans certains aliments, comme le hareng, le bœuf et l’agneau, les graines de tournesol, le fromage et d’autres produits .May 25, 2014 — The most stable oxidation state is one that fills or half-fills an atom’s electron shell. Remember, shells don’t neatly stack on top of each other, so valence (and oxidation state) may not be the same as the total number of electrons in the outer shell.Zinc is a transition metal and is present in the d block in the periodic table. 30 is the atomic number of zinc.Hence, the electronic configuration on zinc is \(1s^22s^22p^63s^23p^63d^{10}4s^2 \).. The valence shell of the element i.e. 4s contains 2 valence electrons.; This means that zinc can lose 2 electrons present in the 4s shell to .valency of zincSet 30, 2018 — Valence, or Valency, describes how easily an atom or radical can combine with other chemical species.
valency of zinc|Valency
PH0 · Zinc Valence Electrons
PH1 · Zinc (Zn)
PH2 · Zinc
PH3 · Valency Chart (Valency Table of Chemical Elements)
PH4 · Valency
PH5 · Valences of the Chemical Elements
PH6 · How to Find the Valence Electrons for Zinc (Zn)
PH7 · How many valence electrons does zinc have?
PH8 · How Many Valence Electrons Does Zinc (Zn) Have?
PH9 · Chemistry of Zinc